Study Overview
Group 1: Non-ambulatory
no age restriction
Group 2: Ambulatory
age 8 to less than 18 years old
The study will evaluate the safety and efficacy of systemic gene delivery in males with DMD. This is a randomized, double-blind, placebo-controlled study of delandistrogene moxeparvovec, of which the total duration of participation is expected to be approximately 128 weeks (30 months).
Purpose of Study
 ENVISION is a phase 3 clinical study to evaluate the safety and effectiveness of an investigational gene therapy in males with Duchenne muscular dystrophy. Both ambulatory and non-ambulatory individuals may be considered for ENVISION.
ENVISION is a phase 3 clinical study to evaluate the safety and effectiveness of an investigational gene therapy in males with Duchenne muscular dystrophy. Both ambulatory and non-ambulatory individuals may be considered for ENVISION.
Duchenne muscular dystrophy (also referred to as Duchenne or DMD) is a genetic disorder that primarily affects males and is caused by a specific genetic mutation (error) in the gene that codes for dystrophin.
Dystrophin is a protein that plays a key role in the function of muscle cells and protects them from damage as muscles contract and relax. These mutations in the dystrophin gene lead to a lack of dystrophin protein in muscles. Without enough dystrophin, muscles gradually grow weaker.
There is currently no cure for Duchenne. Researchers are currently investigating ways to modify disease progression. One approach is gene therapy. This type of therapy adds a new gene to the body, with the hope of treating the underlying cause of disease.
About ENVISION
The ENVISION Study is testing an investigational drug, called delandistrogene moxeparvovec (previously referred to as SRP-9001), to see whether it is safe and effective in individuals with Duchenne. Delandistrogene moxeparvovec is a gene therapy treatment being developed to treat the cause of Duchenne. The aim of gene therapy for Duchenne is to add a new gene inside the muscle cell. Once the delandistrogene moxeparvovec dystrophin gene is inside the muscle, researchers believe it may teach the cells to make delandistrogene moxeparvovec dystrophin protein in hopes to promote muscle function.
How gene therapy works:
Gene therapy comprises three main building blocks: a vector, promoter, and transgene. Gene therapy can be given as a one-time administration by intravenous (IV) infusion – a small tube inserted by a needle will deliver a slow ‘drip’ of study medication into a vein in the arm or leg.
To better understand the building blocks of this gene therapy, picture a ship out at sea heading toward land. Like the ship, when the study drug is introduced to the body it is heading toward certain cells.

- The vector is the ship itself, it carries the instructions to make the desired protein through the body and aims to deliver it into the muscle cell like a ship reaching land.
- The vector used in this study is known as rAAVrh74.
- The dystrophin transgene is like cargo inside a ship. It is a gene made of DNA that teaches the muscle cells how to make the desired protein. The transgene is being carried inside the vector to the muscle cell.
- The promoter functions like the captain of a ship. Like a captain commanding orders, the promoter aims to instruct the muscle cells to produce the protein.
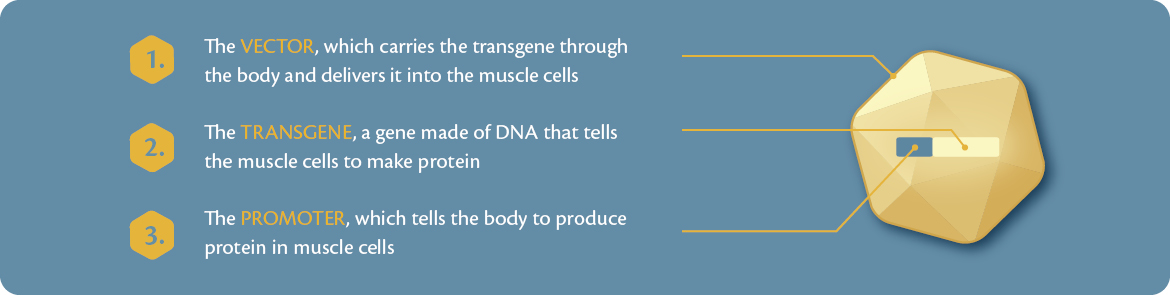
About the Study
Study Name: ENVISION
Study Official Title: A Phase 3, Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Transfer Therapy Study to Evaluate the Safety and Efficacy of SRP-9001 in Non-Ambulatory and Ambulatory Subjects With Duchenne Muscular Dystrophy
Study Number: SRP-9001-303
ClinicalTrials.gov: https://clinicaltrials.gov/study/NCT05881408
Status: Recruiting
Locations: Refer to clinicaltrials.gov for enrolling centers.
Participate
 The study will enroll individuals into two groups:
The study will enroll individuals into two groups:
- GROUP 1: Non-ambulatory male individuals with Duchenne with continuous wheelchair use for at least 6 months. There is no age requirement for Group 1.
- GROUP 2: Ambulatory male individuals with Duchenne, age 8 years to less than 18 years old at time of screening.
Study Eligibility:
- Have been diagnosed with Duchenne based on clinical findings and prior genetic testing. Please note, genetic mutations fully contained between exons 18 to 79 (inclusive) are eligible.
- Have been on a stable dose of any oral corticosteroids for at least 12 weeks, or have not been on corticosteroids for at least 12 weeks prior to the Screening visit.
- Do not have elevated antibodies to rAAVrh74.
- Have a parent, legal guardian or caregiver who can accompany him at each visit and offer support throughout the study.
There are additional requirements for participation that will be reviewed with you and your child during the screening process.
Travel and Reimbursement Policy
During participation in the study, our travel and reimbursement program consists of a range of services at no cost to study subjects. If you need help getting to and from the study center, we can organize transportation – whether by taxi, bus, plane or rail. Arrangements can also be made for accommodations for longer visits or overnight stays. Meals and other reasonable expenses incurred while visiting the study center will be reimbursed. Subject to local regulations.
Cross border participation and relocation services of any kind, including support of Visa applications, will not be supported by the Sponsor. Individual study investigators may consider patients outside of their countries on a case-by-case if:
- The participant is an established patient receiving regular care at the trial site;
- There are no restrictions to travel between the country of residence and country in which the trial site is located;
- The Investigator confirms that there are no barriers to direct communication with the patient and/or caregivers (ie. on-site translator is not required);
AND
- The participant lives in a nearby country where the ENVISION study IS NOT being conducted;
OR
- The participant lives in a nearby country where the ENVISION study IS being conducted, but participant is in closer proximity to the trial site.
SareptAlly
SareptAlly is a clinical trial patient matching service for Sarepta studies.
If you are interested in considering participation click here and schedule a call with a SareptAlly Patient Navigator who will help you identify the best clinical trial option and study site location for you and your child, now or in the future.
Schedule a call with a SareptAlly Patient Navigator »
ENVISION Procedures
ENVISION Screening Visit:
Participants who are selected to screen for the ENVISION Study will meet with the study physician and their research team to discuss the study in greater detail and answer questions about participation.
If agreeable, potential participants will be consented and enter the screening period. During the screening period, the study physician will collect medical information on the participant and perform several tests to assess eligibility.
Assessments include:

Blood work | 
Review of medical history | 
Performing some functional |
If the results of the screening visit show that the participant is eligible, they will be invited back to the study center for study participation. There are additional assessments that may be required.
Study participation in ENVISION consists of 128 weeks (30 months), divided into three parts:
Screening Period: The screening period will consist of several visits. During this time individuals will be screened for eligibility to participate in the study (some outlined above).
Part 1 consists of about 72 weeks. Participants will receive study drug (active or placebo) on Day one of Part 1. Study assessments will continue throughout this period. At the end of 72 weeks, the main study endpoint of upper limb function and safety, will be assessed in all participants.
Part 2 following Part 1, will consist of 52 weeks. Participants who did not receive active study drug in Part 1, will receive the active study drug at the beginning of Part 2. Study assessments will continue through the remainder of the study.

ENVISION Study Visits:

Participants will receive two infusions (active study drug or placebo), one at the beginning of the study (Part 1), and one at the beginning of the second part of the study (Part 2) | 
Study visits will include in clinic visits, as well as visits that can be performed remotely, or over the telephone. The study doctor will discuss which visits can be performed remotely. | 
Participants will visit a study center for infusions, cardiac MRI scans, heart monitoring and imaging, periodic blood draws, and muscle biopsies (at certain sites). |

Functional assessments (upper body tests for all participants; standing, walking, jumping tests for ambulatory participants), vital signs, lung function tests, urine analysis, and physical examinations will also be performed at the study center. | 
Completion of Duchenne-related questionnaires, and usage of wearable devices for tracking movement, will also be required for this study. | 
Additional visits may be scheduled if required – this will be decided by the study doctor. More information on the schedule of study visit activities will be provided by the study center.
|
What is a randomized placebo-controlled study?
Randomized, placebo-controlled means that each study participant will be picked randomly, by chance (like tossing a coin) to receive either active study drug or “Placebo.” Placebo is made to look just like the study drug, but it will not contain any active drug. Neither participant nor the study doctor will know if they have been assigned the active study drug or placebo. During the first part of the study, participants will have a 50% chance of receiving active study drug. All eligible participants will have the opportunity to receive active study drug at the start of the second part of the study.
Researchers use a placebo to compare how safe and how well the study drug works in individuals treated with the drug, versus those receiving placebo and standard of care.
How will the study drug be given?
Participants will receive an infusion at Day 1 of the first part of the study, and at Day 1 of the second part of the study. The infusion will either contain active study drug or placebo based on the randomization assignment at the start of the study. Both the active study drug and placebo will be given as a single intravenous infusion – a small tube inserted by a needle will deliver a slow ‘drip’ of study medication into a vein in the arm. The infusion will last approximately 1–2 hours. After the infusion, the study doctor will monitor the participant.
Part 1; Day 1 | Part 2; Day 1 | |
 Randomized to active study drug | Study Drug | Placebo |
 Randomized to Placebo | Placebo | Study Drug |
During the first part of the study participants will have a 50% chance of receiving active study drug. All eligible participants will have received active study drug by the start of Part 2.
Frequently Asked Questions
-
What is a placebo, what is a placebo group, and why are they being used?
Placebo is made to look just like the active study drug, but it will not contain any active substance. Researchers use a placebo to compare how safe and how well the study drug works in individuals treated with the drug, versus those receiving placebo and standard of care.
-
Why is it important to study gene therapies over a long period?
Gene therapies are designed to be long-lasting. Because we do not know how long the study drug will remain active, it is difficult to predict how people will respond. Study participants are therefore assessed over several years, to determine the effectiveness of the drug, and to see if they develop any complications after the infusion. Evaluating study participants over a long period can also protect the community. For example, finding any safety issues early may provide researchers with important information for study participant safety.
-
Are clinical research studies safe?
Clinical research studies are performed according to strict government and ethical guidelines. These guidelines help to ensure that participants’ rights are protected while information about the investigational drug is collected. All study participants are supported by a dedicated team of healthcare professionals, each of whom is committed to the safety and well-being of those involved.
-
What is an antibody and why would someone have antibodies to rAArh74?
Antibodies are proteins in the blood that the body develops to help fight off an infection. Antibodies are an important part of building immunity. Once they develop, antibodies often remain (to some extent) in a person’s body and could help fight off the same or similar infection in the future.
A participant will not be eligible for ENVISION due to having elevated antibodies to rAAVrh74. Because AAVs are found in nature, it’s possible that a person has been exposed to the AAV virus and formed antibodies. This is known as seroprevalence. If that’s the case, antibodies will be present prior to delivering gene therapy. These antibodies can potentially hinder the effectiveness of the study drug and pose a safety concern if the immune system recognizes the vector as a virus and attacks it, similar to how the immune system works against naturally-occurring viruses.
Participants will be tested for antibodies to rAAVrh74 via blood work during the screening process, and again before Part 2 of the study to see if they remain antibody negative. To learn more about antibodies and seroprevalence click here.
-
Will I be compensated for taking part in this study?
Participants and family members will not be paid for taking part in this study. However, transportation related to participation, accommodations, and all reasonable expenses will be reimbursed. Further details regarding travel arrangements and reimbursement will be provided at the study site.
-
Can someone travel to another country to participate?
Cross border participation and relocation services of any kind, including support of Visa applications, will not be supported by the Sponsor. Individual study investigators may consider patients outside of their countries on a case-by-case if:
- The participant is an established patient receiving regular care at the trial site;
- There are no restrictions to travel between the country of residence and country in which the trial site is located;
- The Investigator confirms that there are no barriers to direct communication with the patient and/or caregivers (ie. on-site translator is not required);
AND
- The participant lives in a nearby country where the ENVISION study IS NOT being conducted;
OR
- The participant lives in a nearby country where the ENVISION study IS being conducted, but participant is in closer proximity to the trial site.
-
Do I need to ask my regular doctor before participating in this study?
Yes, you will need to inform your regular doctor you are taking part in this study. This is a requirement for participation in ENVISION.
-
Will I have to switch doctors?
No. The ENVISION study provides study-related care only.
Clinical research studies do not provide extended or comprehensive healthcare. At the end of the study, study participants will return, in full, to their regular doctor for care.
-
Are there side effects from the study drug delandistrogene moxeparvovec?
There are risks, discomforts, and inconveniences associated with research studies. Because delandistrogene moxeparvovec is still in clinical development, there may be side effects that are not known at this time. Participants may also experience side effects from the medical tests and procedures. During the informed consent process, the study team will discuss potential side effects and discomfort that may occur while on the study.
If a study participant does experience side effects, contact the study doctor at any time to discuss the best course of action.
-
What happens at the end of the ENVISION Study?
At the end of the study, participants may be eligible to enter a long-term extension study, which will assess the safety and effectiveness of the study drug. Further details will be given to you by the study team.
-
Can I leave the study early?
It is voluntary to join the study. Participants can leave the study at any time without giving a reason. Your decision will not affect any future medical care that you will receive. However, once you have received the infusion, it is in your best interest to allow the study doctor to continue to monitor your health for the remainder of the study.
-
Who will have access to the information collected during the study?
Personal identifiable information, such as names and addresses, will not be given to anyone who is not directly associated with this study, except with your permission or as required by law. Any information gained from this study may be used for publishing results. However, this information will be combined with other data and will not be used to identify participants.
-
How do I take part?
If you are interested in the ENVISION study, you can schedule a meeting with a SareptAlly Patient Navigator to learn more and see if you are eligible. If you have general questions about the trial, trial locations, or participation, you may also contact the Patient Navigator at SareptAlly@Sarepta.com. Please note, if you would like to get in touch with a participating study center directly, contact information is available on clinicaltrials.gov.
-
What should I consider before taking part?
Taking part in clinical research requires careful thought and commitment. We encourage you to review all the documents provided, which give important information about the different components of delandistrogene moxeparvovec gene therapy, the reasons why it is being studied, and the risks and benefits of study-related procedures. After you have read these documents, we strongly recommend that you discuss your questions and concerns with the study doctor.
-
Why should I take part?
A clinical research study is an important step toward finding potential future treatments for individuals with Duchenne. If you decide to take part, you’ll be helping researchers understand this disease better and playing an important role in ongoing efforts to improve healthcare.
