OVERVIEW: PART A
OVERVIEW: PART B
The goal of Part B is to examine treatment with the study drug every 4 weeks at the doses selected based on data from Part A. There are two cohorts in Part B: (1) Previously Treated participants who previously received the investigational SRP-5051 treatment in Part A of this study or in study 5051-102 and (2) Treatment-Naïve participants newly enrolled in the study who have not previously received the investigational SRP-5051 treatment.
The study is closed to enrollment.
Participant Eligibility Criteria for Participants Treatment-Naïve to SRP-5051
Your child may be eligible to participate in Part B of the Momentum trial if he meets the following eligibility criteria:
- Ambulatory and non-ambulatory boys with DMD who are 7-21 years old and Treatment-Naïve
- Has a genetic diagnosis of DMD and an out-of-frame deletion mutation of the DMD gene amenable to exon 51-skipping treatment. Talk to your doctor if you are unsure.
- Has been on a stable dose of corticosteroids for at least 12 weeks, or has not received corticosteroids for at least 12 weeks prior to receiving SRP-5051
- Has stable lung (breathing) and heart function.
- Has not received treatment with any exon-51 skipping therapy within 4 weeks prior to Screening, or with any experimental gene therapy for the treatment of DMD at any time
- Does not have hypomagnesemia at Screening, serum creatinine above the upper limit of normal at Screening, or any other abnormal electrolyte values considered clinically significant by the study doctor at Screening.
Additional requirements for participation will be reviewed with participants and their families during the screening process.
Frequently Asked Questions
-
What is an open label study?
Open-label means that the study doctor, nurses, caregivers, Sponsor, and you will all know that you are receiving study drug, and at what dose level.
-
What does multiple ascending dose mean?
Multiple means that all participants will receive doses of the study drug once every 4 weeks throughout the study. Ascending dose means that there are different study drug dose levels in Part A that increase from one dose group to the next.
-
What does dose expansion mean?
In Part B of the study, participants will be assigned to 1 of 2 dose groups and subsequently receive doses of study drug based on their body weight.
-
How many boys will be enrolled in this study and where is it being run?
Approximately 60 participants are planned to be enrolled in this study (~30 participants who previously received SRP-5051 and ~30 participants who are treatment-naïve to the study drug), with clinical trial sites planned in the United States, Canada and Europe. Additional information on the location of clinical trial sites may be found on ClinicalTrials.gov.
-
How long is the study?
Duration of participation for participants who enroll in Part B of the study is expected to last approximately 2 years.
-
Will I be paid for participating?
Participants will not be paid for participation in this study. However, reasonable travel costs associated with participation in the study will be prepaid or reimbursed by Sarepta in accordance with the approved study travel policy. Information will be provided by the study site.
-
What are some of the activities that will be required to take part in this study?
Enrolled participants will visit study sites for monthly dosing, functional assessments, medical testing, and muscle biopsies at the applicable time points over the course of the study. Some visits will require overnight stays at the study site. Participants will undergo safety labs at each visit. Additional information will be provided by the study site.
-
Why do you need to collect biopsies?
This study will measure the change in the amount of dystrophin protein in skeletal muscle tissue after study treatment. Because we are testing to see if SRP-5051 increases levels of dystrophin in skeletal muscle tissue through exon-skipping, the only way to show SRP-5051 is working as intended is to test skeletal muscle tissue directly. This cannot be studied by testing blood or urine, for example. More information will be provided by the study site.
-
What risks are associated with this study?
As with all clinical studies, there can be risks associated with possible side effects of taking the study drug and with the standard medical tests carried out as part of the study at each visit. Information on the possible side effects that may be experienced in the study is available in the consent form and should be discussed with your study doctor.
-
What are some benefits to being a part of this clinical trial?
The potential benefits of SRP-5051 in people with DMD are unknown. Even if you do not benefit from being in this study, we might learn something that could advance research and help others.
SareptAlly is a clinical trial participant matching service for Sarepta-sponsored studies.
A Patient Navigator can help you understand your clinical trial options.
Exon Skipping for Duchenne
About Duchenne
Duchenne is a rare, life-shortening genetic disorder that affects boys and causes their muscles to break down and lose strength over time. Duchenne is caused by specific errors (mutations) in the gene that codes for dystrophin. Dystrophin is a protein that plays a key role in the function of muscle cells and protects them from damage as muscles contract and relax. These mutations in the dystrophin gene lead to a lack of dystrophin protein in muscles. Without enough dystrophin, muscles gradually grow weaker until they can’t move at all, and eventually breathing and heart function are lost.
About SRP-5051
SRP-5051 is an investigational drug designed to treat Duchenne in patients who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 51. It uses a technology called exon skipping.
About Exon Skipping
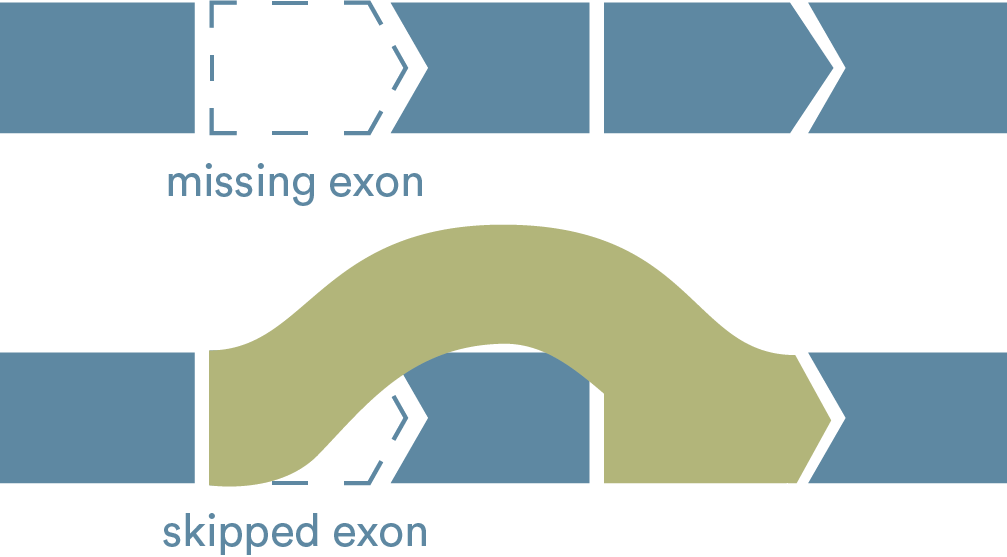 Exon-skipping technologies strive to address the underlying issue with Duchenne—a lack of the protein dystrophin. Many people with Duchenne have a genetic mutation in which one or more exons in the dystrophin gene are missing. This causes errors in the instructions for making dystrophin, leaving the body unable to produce the protein.
Exon-skipping technologies strive to address the underlying issue with Duchenne—a lack of the protein dystrophin. Many people with Duchenne have a genetic mutation in which one or more exons in the dystrophin gene are missing. This causes errors in the instructions for making dystrophin, leaving the body unable to produce the protein.
Exon skipping tells the body to hide an exon next to the missing piece so the whole section can be skipped over and the remaining exons can fit together. The goal of exon skipping is to allow the body to make a shorter form of the dystrophin protein.
Trial Locations
The MOMENTUM Study has sites planned in the United States, Canada, United Kingdom, and EU. To see a current list of participating global study centers, visit clinicaltrials.gov

